
Vodafone Foundation donates €30,000 to support Mozambique over Cyclone Chido
Vodafone Foundation has pledged €30,000 to Save the Children in response to the devastation caused ...
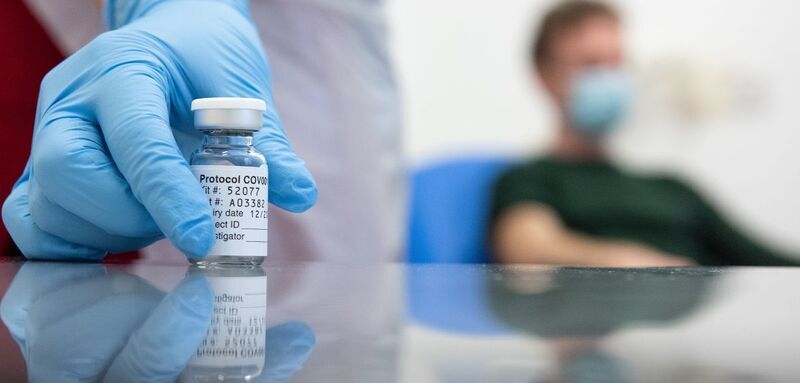
The University of Oxford, in collaboration with AstraZeneca plc, announced interim trial data from its Phase III trials that show its candidate vaccine, ChAdOx1 nCoV-2019, is effective at preventing COVID-19 (SARS-CoV-2) and offers a high level of protection.
Professor Andrew Pollard, Director of the Oxford Vaccine Group and Chief Investigator of the Oxford Vaccine Trial, said “These findings show that we have an effective vaccine that will save many lives. Excitingly, we’ve found that one of our dosing regimens may be around 90% effective and if this dosing regimen is used, more people could be vaccinated with planned vaccine supply. Today’s announcement is only possible thanks to the many volunteers in our trial, and the hard working and talented team of researchers based around the world.”
Professor Sarah Gilbert, Professor of Vaccinology at the University of Oxford, said “The announcement today takes us another step closer to the time when we can use vaccines to bring an end to the devastation caused by SARS-CoV-2. We will continue to work to provide the detailed information to regulators. It has been a privilege to be part of this multi-national effort which will reap benefits for the whole world.”
Following the trial reaching the target for interim analysis, the independent Data and Safety Monitoring Board (DSMB) recommended that the team at Oxford conduct its first analysis on all the cases with data locked on 4 November 2020.
These preliminary data indicate that the vaccine is 70.4% effective, with tests on two different dose regimens showing that the vaccine was 90% effective if administered at a half dose and then at a full dose, or 62% effective if administered in two full doses.
Additional cases are expected to accrue by the time of the final analysis and future analyses will determine the duration of protection. No serious safety events related to the vaccine have been identified.
Oxford will now support AstraZeneca in submitting both the interim Phase III efficacy data and the extensive safety data to all regulators across the world, including in the UK, Europe and Brazil for independent scrutiny and product approval, including for emergency use. Many of these regulators have been reviewing the trial data on a rolling basis during the trial.
In parallel, Oxford is submitting the full analysis of the Phase III interim data for independent scientific peer review and publication.
The coordination of the program and execution of the trials in the UK would not have been possible without the support of the National Institute for Health Research and UKRI.
These data also suggest that this half dose and full dose regimen could help to prevent transmission of the virus, evidenced by lower rates of asymptomatic infection in the vaccinees, with further information to become available when trial data are next evaluated.
The interim Phase III data builds on Oxford’s phase I/II peer-reviewed trial results which have shown that the vaccine induces strong antibody and T cell immune responses across all age groups, including older adults, and has a good safety profile.
The clinical trials, enrolling over 24,000 participants from diverse racial and geographical groups in the UK, Brazil and South Africa, will now continue to final analysis. Further trials are being conducted in the United States, Kenya, Japan and India and the trial team expect to have under 60,000 participants by the end of the year. These trials will provide regulators with further information about the efficacy and safety of the Oxford candidate vaccine, including its ability to both protect against and stop the transmission of COVID-19.
Oxford University’s collaboration with AstraZeneca has been crucial to the successful development of the vaccine and vital for its global manufacturing and distribution across the world. AstraZeneca already has international agreements in place to supply three billion doses of the vaccine, with access being built through more than 30 supply agreements and partner networks.
Professor Louise Richardson, Vice-Chancellor at the University of Oxford, said “This is a great day for the University of Oxford and for universities everywhere. Pushing at the frontiers of knowledge with partners across the globe and putting our extraordinary brainpower in service to society, is what we do best.”
Pascal Soriot, Chief Executive Officer, AstraZeneca, said “‘Today marks an important milestone in our fight against the pandemic. This vaccine’s efficacy and safety confirm that it will be highly effective against COVID-19 and will have an immediate impact on this public health emergency. Furthermore, the vaccine’s simple supply chain and our no-profit pledge and commitment to broad, equitable and timely access means it will be affordable and globally available supplying hundreds of millions of doses on approval.”
Vodafone Foundation has pledged €30,000 to Save the Children in response to the devastation caused ...
The European Commission has adopted a decision to disburse €1 billion in loans to Egypt following ...
Opening the Helwan University clinic brings the total number of Safe Women Clinics to 48 ...


اترك تعليقا