
Report highlights 2 Egyptian projects among major clean energy ventures in N.Africa
A report by Energy Capital & Power highlighted two Egyptian projects – Suez Wind Power ...
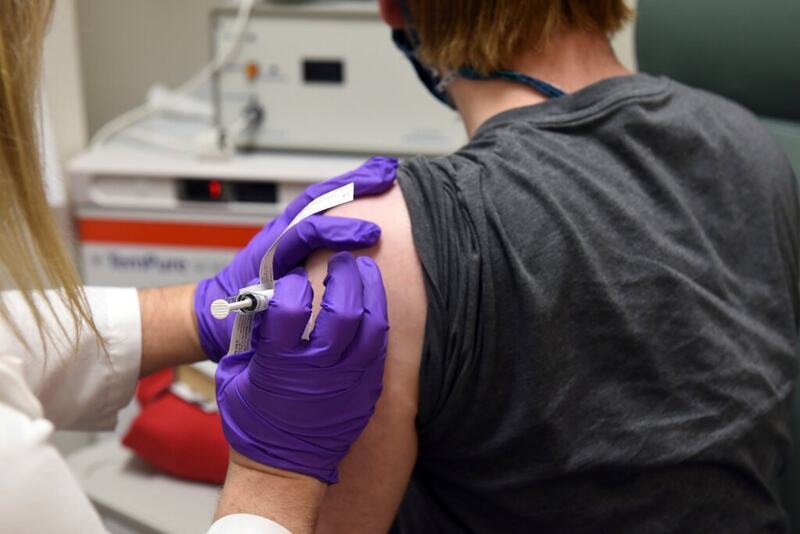
As nations around the world race to lock up coronavirus vaccines even before they are ready, the Trump administration on Wednesday made one of the largest investments yet, announcing a nearly $2 billion contract with Pfizer and a German biotechnology company for 100 million doses by December.
The contract is part of what the White House calls the Warp Speed project, an effort to drastically shorten the time it would take to manufacture and distribute a working vaccine. So far, the United States has put money into more than a half dozen efforts, hoping to build manufacturing ability for an eventual breakthrough.
Europe has a parallel effort underway. Germany recently took a 23 percent stake in a German firm, CureVac, that President Trump once tried to lure to American shores in hopes that its vaccine, if successful, would be distributed in the United States first.
A European-led fund-raising effort in May brought $8 billion in pledges from the world’s governments, philanthropists and leaders for coronavirus vaccine research, even with the United States sitting out the conference.
China, meantime, has militarized the effort: Researchers associated with the Academy of Military Medical Sciences have developed one of China’s leading vaccine candidates, and another Chinese firm, Sinopharm Group, announced in June that it was beginning Phase 3 trials in the United Arab Emirates.
The Pfizer contract, an agreement to ensure the pharmaceutical giant has a market for its work, is the biggest splash yet by the Americans. No vaccine has yet been developed, and it is not clear whether the Pfizer version will work. But if the vaccine being produced by Pfizer and BioNTech, the German firm, proves to be safe and effective in clinical trials, the companies say they could manufacture those first 100 million doses by the end of the year.
Under the arrangement, the federal government would obtain that first batch for $1.95 billion, or about $20 a dose, with the rights to acquire up to 500 million more, or 600 million total. Americans would receive the vaccine for free. Before it could be distributed, it would need emergency approval by the Food and Drug Administration. But the U.S. government does not pay the nearly $2 billion until the drug is approved and the first 100 million doses are delivered.
“Depending on success in clinical trials, today’s agreement will enable the delivery of approximately 100 million doses of vaccine being developed by Pfizer and BioNTech,” Alex M. Azar II, the health secretary, said in a statement announcing the deal.
On Monday, Pfizer and AstraZeneca, a British-Swedish drug company developing a potential vaccine with Oxford University, released data suggesting that their vaccines could stimulate strong immune responses with only minor side effects.
But unlike AstraZeneca, which has also obtained funding from the U.S. government, Pfizer did not receive a contract for its earlier research and development efforts — only for the doses and their distribution.
By refusing funding up until now, Pfizer was able to avoid drawn-out contractual negotiations and get its vaccine to trials, company officials say.
“We didn’t accept the federal government funding solely for the reason that we wanted to be able to move as quickly as possible with our vaccine candidate into the clinic,” John Young, Pfizer’s chief business officer, said on Tuesday at a congressional hearing with executives from five vaccine manufacturers.
Pfizer and BioNTech are developing a vaccine candidate that uses genetic material from the virus, known as messenger RNA, to stimulate the immune system without making the recipient sick. The technology can create a vaccine quickly, but has not yet produced one that has been approved and marketed.
Moderna, a Massachusetts biotech company, received $483 million from the U.S. government for its vaccine development and is also using mRNA technology. By putting the might of an industry giant behind it, Pfizer is making the technology mainstream.
The lack of a track record has prompted some skepticism about this approach, but Dr. Kathrin Jansen, a senior vice president and the head of vaccine research and development at Pfizer, dismissed the criticism.
A report by Energy Capital & Power highlighted two Egyptian projects – Suez Wind Power ...
The opening concert of the Annual Meeting 2025 in Davos-Klosters will address the pressing issues ...
Juhayna Food Industries proudly announced that its agricultural arm, El Enmaa for Agricultural Development, has ...


اترك تعليقا