
Report highlights 2 Egyptian projects among major clean energy ventures in N.Africa
A report by Energy Capital & Power highlighted two Egyptian projects – Suez Wind Power ...
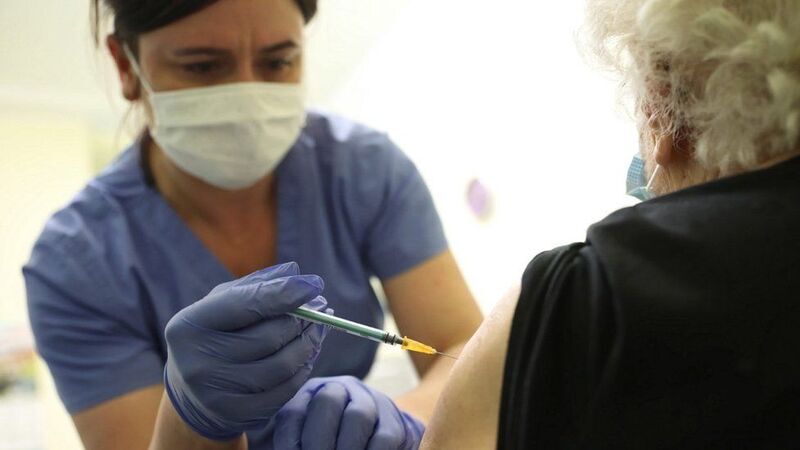
Novavax published late Thursday positive data from the UK phase 3 study of its COVID-19 vaccine candidate, showing it to be 89.3% effective in preventing coronavirus in participants.
The study was conducted during the period the new COVID-19 variant was first observed in Kent and began to circulate widely, with today’s results showing it was effective against the variant during the phase 3 trial.
Thanks to the work of the government’s Vaccines Taskforce, the UK has secured 60 million doses of Novavax’s vaccine to be delivered in the second half of this year, if approved for use by the Medicines and Healthcare products Regulatory Agency (MHRA), who will assess whether the vaccine meets robust standards of safety, effectiveness and quality.
Last August Novavax announced plans to manufacture the bulk of the vaccine using FUJIFILM Diosynth Biotechnologies’s facilities in Billingham, Stockton-on-Tees. This will ensure that, once available, the vaccine can be supplied to the British public as soon as possible.
Business Secretary Kwasi Kwarteng said “The results from the UK trial of Novavax’s vaccine look extremely promising, and I welcome the news that the company is planning to submit its data to the regulators.”
“The UK moved quickly to procure 60 million doses from Novavax and I’m pleased to confirm the bulk of the vaccine will be manufactured on Teesside and delivered during this year, if approved for use.”
“From the scientists and researchers to the thousands of UK trial volunteers, I am enormously grateful to everyone who is playing their part in this truly national effort to defeat this virus once and for all.”
Health Secretary Matt Hancock said “This is positive news and, if approved by the medicines regulator, the Novavax vaccine will be a significant boost to our vaccination program and another weapon in our arsenal to beat this awful virus.”
“I’m proud the UK is at the forefront of another medical breakthrough and I want to thank the brilliant scientists and researchers, as well as the tens of thousands of selfless volunteers who took park in clinical trials.”
The NHS stands ready to roll this vaccine out as quickly as possible to those most at risk if it is authorized.
Vaccines Minister Nadhim Zahawi said “Having taken part in Novavax’s vaccine trial myself, I am particularly thrilled to see such positive results. I want to thank the thousands of trial volunteers, without whom these results would not have been possible.”
“It will now be for the regulator to do its crucial work in assessing the efficacy and safety of this vaccine, but if approved it will be a further boost to our vaccination program.”
Novavax’s candidate differs from those currently being used in the UK, combining an engineered protein from the virus that causes COVID-19 with a plant-based ingredient to help generate a stronger immune response. Having a diverse portfolio of vaccines increases the chances of ensuring there is a vaccine available for everyone across the UK.
The data published today come from more than 15,000 people who were recruited through the National Institute of Health Research vaccine registry, which was launched in July 2020 to support the UK’s efforts to deliver vaccines for COVID-19. Nearly 4,000 people in the study were over the age of 65.
Through the Vaccines Taskforce, the UK has secured early access to 367 million doses of 7 of the most promising vaccines so far. To date, the UK government has invested over £230 million into manufacturing a successful vaccine.
The UK was the first country in the world to procure, authorize and then deploy both the Oxford/AstraZeneca and Pfizer/BioNTech vaccines.
Production of the Oxford University/AstraZeneca vaccine started last autumn where the bulk of the vaccine for the UK is being made in Oxfordshire and Staffordshire, with filling into vials taking place in North Wales.
In total, more than 7.4 million people across the UK have now had a least one dose of the vaccine.
A report by Energy Capital & Power highlighted two Egyptian projects – Suez Wind Power ...
The opening concert of the Annual Meeting 2025 in Davos-Klosters will address the pressing issues ...
Juhayna Food Industries proudly announced that its agricultural arm, El Enmaa for Agricultural Development, has ...


اترك تعليقا